Area: Chemistry and Art
Grade Level: Primary school (age 9 -11)
Course: STEAM
Timeframe: 30-40 min
Lesson Overview
In this lesson you will build an artwork on which you will grow salt crystals. You will keep a log over during the time the salt crystals are forming.
Objectives
Upon completion of this Lesson students will be able to:
- Grow salt crystals and know how they are formed
- Understand what atoms are
- Keep a log of their experiment
Material/ resources
Please number all the instructional tools/resources material you will use in your lesson with a short title and source for copyright issues (include references were needed in the relevant section).
- Pan/kettle
- Kitchen Salt (400 gram)
- Water (1 liter)
- Wire/Thread/Yarn
- Toothpicks/skewers
- Bowl
- Wisk
- Optional: colour pigment or dye
Lesson Activities
The salt crystals can be grown in the classroom or at home.
Building of the artwork:
Start with making artwork from wire/thread/yarn which should fit inside the bowl/container you are using. You can also use toothpicks or small wooden skewers or sticks to build your frame for the salt crystals to grow on!
Instructions salt solution:
- Bring water to a boil
- Add the salt and mix well till all the salt is dissolved (be patient, it might take a bit of time! Keep stirring)
- When all the salt is dissolved, pour the water in a transparent container/bowl
- Place your creation of wire/thread/yarn in the water so it is partly submerged or touches the water enough so it can suck up the water
- Place the container on a warm spot (in the sun or near a radiator)

It can take a very long time before you see crystals forming: sometimes two or three weeks. That's why you need a little patience. But you usually see the first crystals appear after a few days. On a warm spot your crystals will grow faster since the water will evaporate faster.
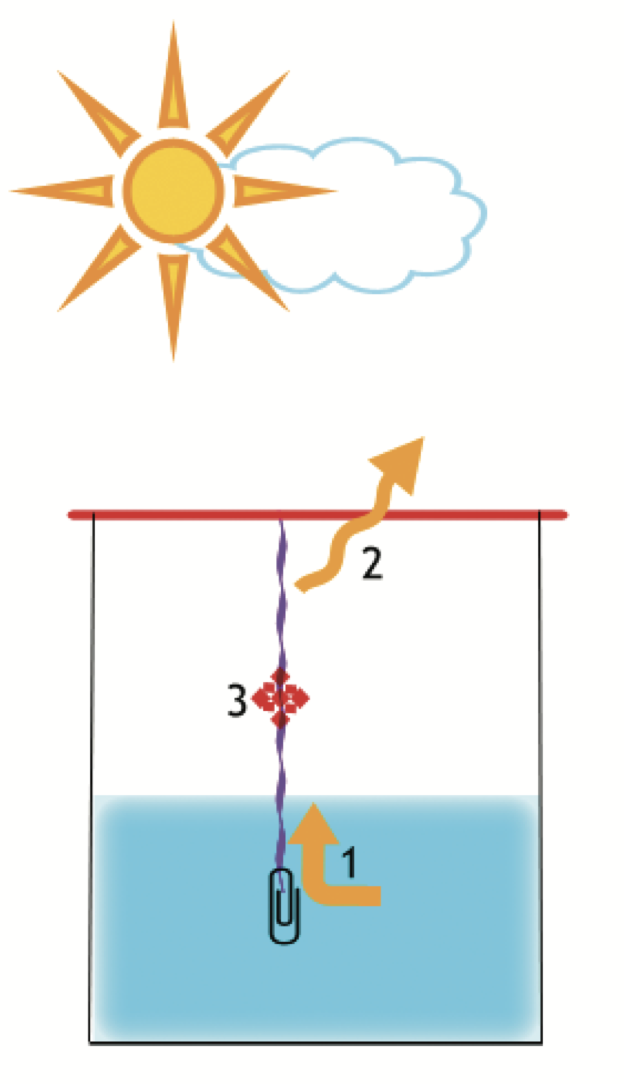
How do salt crystals form?
- The saline solution is drawn into the thread
- The water evaporates due to the heat.
- The salt remains and these become salt crystals
Scientists write very precisely how they conducted their experiments, often referred to as ‘keeping a log’. Keeping such a log is very useful: you will know exactly what you did later on. Now that you are going to build a salt crystal yourself as a scientist, you should of course also make a log! For example, write a piece every day or take pictures of your crystals. You also write down everything that goes wrong in your logbook.
Instructions for teachers
Background information
The story behind your salt crystal
You now know how to make salt crystals, but what actually happens when you do that? Below you can read exactly what happens in your saline solution.
Water and salt are not alike. Salt consists of grains and water is a liquid at normal temperature. When we dissolve the salt in the water, we have a mixture. In that case, the salt particles float in the water.
As long as you use a little bit of salt, salt dissolves well in water. But if you dissolve a lot of salt in water, the water will be "full" at some point - no more salt can be dissolved. We call this a saturated solution. If you add more salt, the extra salt will simply remain in the bottom of your cup as granules.
You can compare it to a classroom where there are chairs, but where there are no children. If the teacher lets a few children into the room, they can all sit on a chair. But when the teacher puts a lot of children in the classroom, at some point all the seats are taken. The children who then remain must stand, just like the salt grains at the bottom of the cup.
After you make the saturated solution, put the solution on a warm one spot, causing the water to evaporate little by little. But because water evaporates, there is also less room for the salt to dissolve! The more water evaporates, the more salt becomes solid salt again. Think of the example of the classroom; if the classroom is full and the teacher removes the chairs one by one, more and more children have to stand.
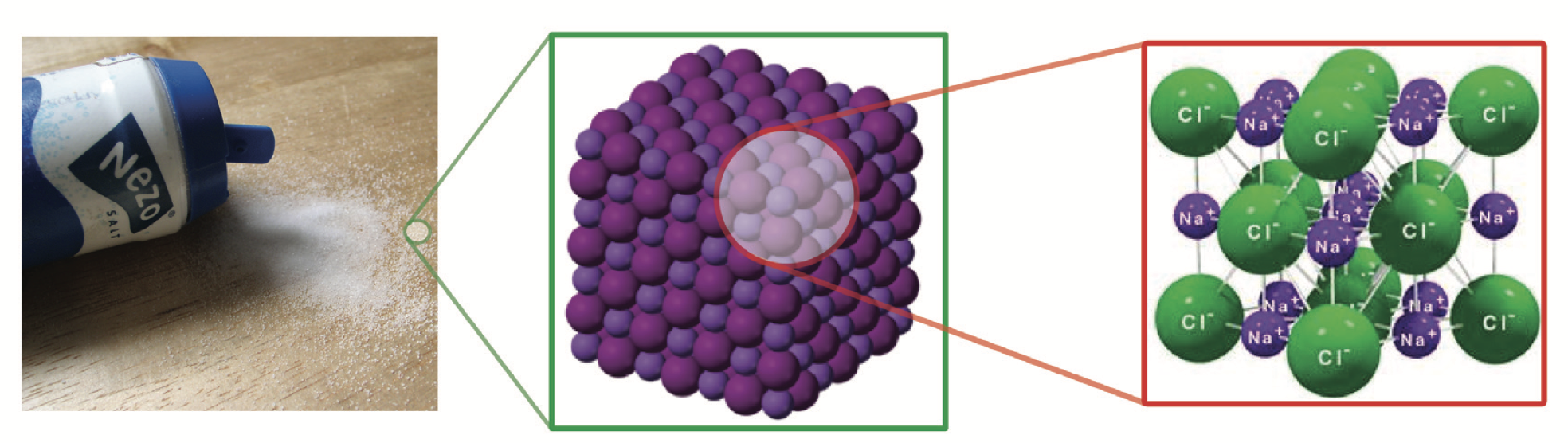
Maybe the children are not happy that they have to stand. But when salt can no longer dissolve, it forms salt crystals. And that's not bad, that's fun! A crystal is a solid that has a very regular structure. Below you see a picture of a salt crystal. Just look: all the balls are neatly attached to each other in the same way. We call these balls ‘atoms’.
But what exactly are atoms? Atoms are the building blocks of everything you see: the whole world around you is made up of atoms. Atoms are enormously small, so small that you can no longer see them even with a microscope. Well, except with the very best microscopes. There are over a hundred different atoms and everything around you is made up of them.
There are two different types of atoms in a salt crystal. We call these sodium atoms and chlorine atoms. They are always sitting next to each other, and also above and below each other, layer after layer after layer. In the picture of the salt crystal, the small spheres are the sodium atoms and the large spheres are the chlorine atoms.
If you make salt crystals by evaporating water, that crystal builds up slowly. One by one the sodium and chlorine atoms join together: atom after atom, layer after layer. The piece of crystal that you see in the picture is just a very small piece of a grain of salt: it contains many more atoms.
In real life, atoms have no color: not purple, not pink, not yellow, not green. We often draw them with a color, but that is because then you can clearly see the difference between the atoms.
Back to the crystals: Crystals are also found in nature. Snowflakes are examples of crystals, but also gemstones such as diamonds are crystals that you can find in nature. In the pictures below you can see a number of beautiful crystals from nature. As you can see, crystals come in different shapes!

Crystals in science
Some scientists find it fun and exciting to look at crystals. But they don't look at the color or the shape, but how the atoms in the crystal are connected. And that differs from substance to substance: the atoms in a salt crystal are attached to each other very differently than the atoms in diamond.
Reference list
So, you now know what happens when you make salt crystals. Moreover, you know that crystals are not only beautiful, but also very useful. Do you want to know more? More information can be found at www.zoutkristallen.nl (in Dutch). Here you can find fun facts and stories about salt, such as where salt comes from and how it ends up on the table. Also here are the logs of other children and you can leave messages in each other's guestbook.