Area: Chemistry
Grade Level: Primary School (from year 1)
Course: Acids and Bases
Timeframe: 30-40 min
Lesson Overview
- DIY kitchen science, to do at school or at home. Almost everything you need can be at home or at the supermarket. Anyone can do it, enjoy!
- two colourful experiments: 1. rainbow skittles and 2. colourful cabbage
Objectives
Upon completion of this Lesson students will be able to:
- be curious to find out how things work, and see science is everywhere around them
- understand some chemistry behind colour changes
- know the difference between acids and bases
Material/ resources
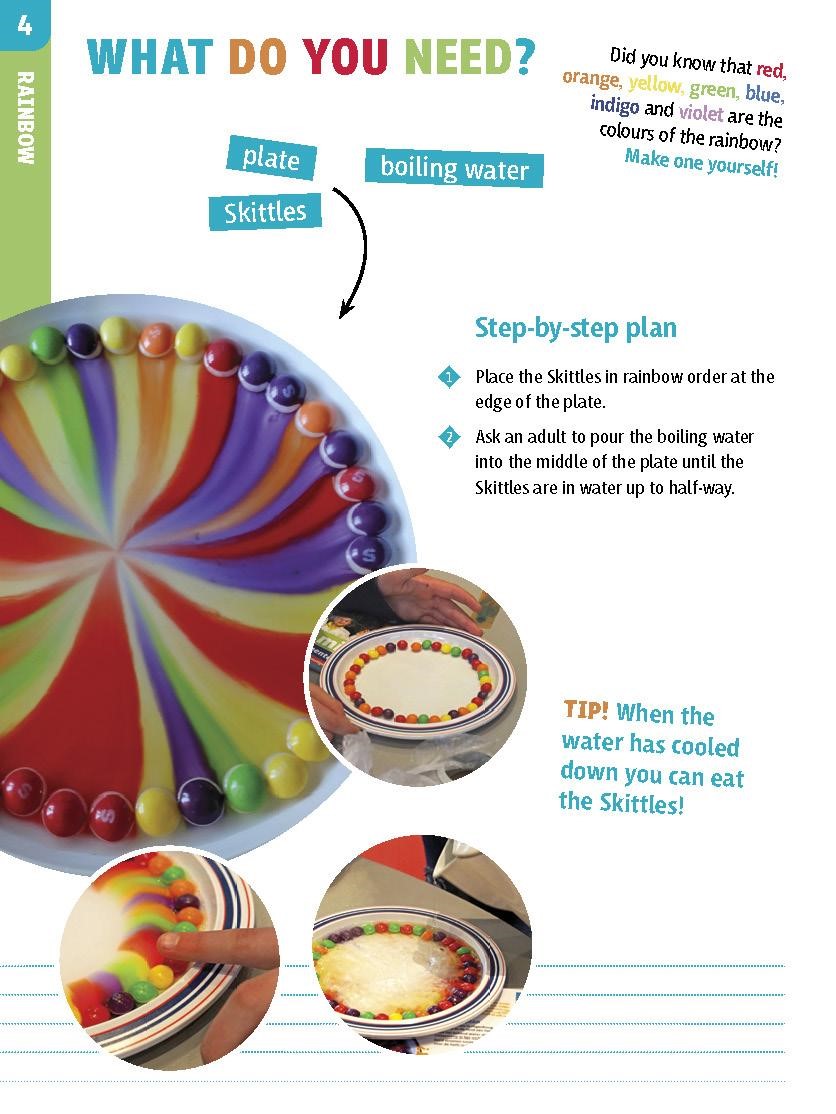
Experiment 1, rainbow skittles:
- Skittles
- plate
- boiling water
Experiment 2, colourful cabbage:
- a red cabbage (fresh or in a jar)
- a tablespoon
- a small bowl
- a pan
- a knife
- liquids you want to test for their acidity
- a glass
Please add the description of each Activity (150 – 200 words) including:
- Setting: the activities can take place either in a classroom, an outdoors place, or in the kitchen. They were in fact designed as experiments to be carried out at home but they could be implemented in various contexts.
- The activities are hands-on are designed based on a problem-based approach and experimentation that requires active engagement by the learners
- The activities are designed to be carried out in small groups (3-4 students) to maximize active engagement. They could be implemented in a classroom with a typical size of 20-25 students, and not more than 6 groups of students per one teacher.
- There is no prior knowledge that is required but observation and simple experiment skills are essential.
- The only materials required are the following for each group of students
- a red cabbage (fresh or in a jar)
- a tablespoon
- a small bowl
- a pan
Lesson Activities
- Make a drawing of a rainbow: what are the colours and in which order?
- Place the Skittles in rainbow order at the edge of the plate (10 mins)
- The teacher pours boiling water in the middle of the plate, until the Skittles are in water up to half-way. (5 min.)
- Describe what is happening and ask how it works. Explanation: The heat of the water causes the dyes on the Skittles to become liquid and mix with the water.The dyes move most easily to places where there is no other dye, which is why the dyes move towards the centre.
- When the water has cooled down you can eat the Skittels :)

 Colourful Cabbage (fresh)
Colourful Cabbage (fresh)
- Cut up part of the red cabbage into small pieces (teacher)
- Put the pieces into a pan and add some water
- Cook the cabbage (teacher)
- Turn off the hob and let it cool down
- When everything has cooled down, drain the red cabbage juice through a sieve into a bowl.
- Skip step 1-5. Drain the red cabbage juice into a bowl or glass.
Now the real experimenting can begin!
Pour a little purple water into a glass and add a liquid that you want to test. For example, water, vinegar, lemon juice, soapy water or baking soda in water. The liquids change from purple to a different colour. They turn green/blue, purple or red. - Also fun: Mixing different colours. What happens now?
This is the most important step in assessing the students learning: what do they observe? Is this what they expected, and why? If not, why is it different?
Summative assessment at end of the lesson
Summative assessment is done through an online science quiz at the end of the lesson where students are asked to respond to various questions around the science concepts introduced in the activities. Below there are 4 indicative questions.
- What causes the color changes? (Peha or acid base)
- An acid can neutralize the base (true or false)
- A base can neutralize an acid (true or false)
- What can the color of an indicator tell you about the substances added to it?
Formative assessment throughout the lesson:
Formative assessment will take place throughout the activities through two ways:
- observations of the ways in which students engage with the activities (e.g., active role with experimentation, enthusiasm, interaction and collaboration with other students)
- whole-group discussion around questions related to the process of experimentation as well as transfer of scientific knowledge to everyday life settings:
- what did you observe?
- why did this happen?
- what would happen if we added more soap or baking soda?
- what happens when people have stomach-pain?
- how is the water in swimming pools tests for its acidity?
- What are some things acids and bases are used to make?
- What makes a weak base?
- What makes a weak acid?
- Why is water important to acids and bases?
- What are strong acids?
- What are weak acids


Please describe how teachers can
The teacher acts as a facilitator walking from group to group checking for understanding through questioning with the group. Important decisions to make include the formation of the groups to ensure balanced interactions and power dynamics and assigning equally agentic roles to the students.
The teacher also provides feedback on the work-in-progress alongside positive reinforcement to each group.
Student scaffolding can be achieved through a series of questioning and assigning of mentoring roles within the group.
Student learning can be examined during this process through questioning as a form of a summative assessment.
Instructions for teachers
Colourful Cabbage explained
The red cabbage juice is what we call an indicator. If you add acids such as citric acid or vinegar, the colour of the juice changes from purple to red because the substances in the red cabbage juice change into other substances with a red colour.
Baking soda and soapy water are the opposite of acids: bases. These substances also react with the red cabbage juice but this time it changes into a blue/ green substance.
If a substance is not an acid or a base, such as water, it does not react, and the juice stays purple. Acids and bases react together to form water. So mixing blue and red will give you purple again.
Want to explore more?
Use this method to measure the acidity of the soil in your neighbourhood.